A characteristic problem associated with cultivation in calcareous soils is the condition called iron chlorosis, a consequence of extreme iron deficiency and whose most characteristic symptom is intervenalchlorosis which is corrected with application of Fe in forms available to plants (Emery, 1982). On the other hand the condition of iron chlorosis isn’t exclusive to calcareous soils, although most of the problems of this type are found in regions with this type of soil (Brown and Jolley, 1989).
Calcareous soils comprise approximately a third of the land surface. The important characteristics of a calcareous soil are a high pH (7 to 9) and a significant content of free carbonates (Gildersleeve and Ocampaugh, 1989). When a plant that lacks certain metabolic abilities grows in calcareous soil, it develops symptoms of iron chlorosis that result because Fe isn’t found in an available form. The means used until now to solve the problem are: local application of salts and chelates of Fe to the plants (application to soil or foliage), artificial modification of the pH of the soil solution (application of organic or inorganic acids) and use of cultivars with the ability to take Fe from soils where the element is unavailable. (Olsen et al., 1987; Chen y Barak, 1982; Emery, 1982).
Iron (Fe) is one of the most deficient micronutrient around the world. The main causes of this deficiency are:
- Calcareous soils
- High soil and water pH
- High concentration of HCO3 (Bicarbonates)
- Wrong application and instability of different fertilizers.
Iron is a micronutrient essential for all higher plants. In general, Fe+2 is the species taken up by plants. Fe+3 therefore have to be reduced at root surface before uptake. In grasses, however, uptake of Fe+3 is of major importance. The formation of complexes and its reaction as a reversible oxidation-reduction reaction system
[Fe+2 <=> Fe+3 + e-] constitute the major metabolic function of iron in plants. Fe is mainly a component of enzymes and proteins.
MAIN ROLE OF FE IN THE PLANT
Iron Fe is most important for the respiration and photosynthesis processes. Iron is also implied in many enzymatic systems like chlorophyll synthesis.
The mobility of iron in the plant is very low.
- Fe is important for the Respiration and Photosynthesis processes.
- Enzymatic Activator
- Chlorophyll Synthesis
- Low mobility in the plant
Absorption and Assimilation of Iron
Fe is present in two oxidation states: Fe+3 ferric and Fe+2 ferrous. In the presence of O2 Fe+2 is rapidly oxidized to Fe+3, which is poorly soluble in water and which precipitates as oxides of Fe; mainly as Fe oxide, Fe hydroxide, Fe phosphate.
 The ions of heavy metals (such as Fe, Zn or Cu) don’t freely cross the cell membrane. The forms that pass are metal chelates. The chelates are synthesized biologically and function to transport metal ions as so-called ionophores. The ionophores specific to iron are known as siderophores (Emery, 1982; Olsen et al., 1981; Kloepper et al., 1980).
The ions of heavy metals (such as Fe, Zn or Cu) don’t freely cross the cell membrane. The forms that pass are metal chelates. The chelates are synthesized biologically and function to transport metal ions as so-called ionophores. The ionophores specific to iron are known as siderophores (Emery, 1982; Olsen et al., 1981; Kloepper et al., 1980).
In many habitats and under many conditions there is competition for Fe among different organisms; the competition is decided by their relative ability to produce siderophores to complex iron (Emery, 1982; Olsen et al., 1981; Kloepper et al., 1980; Murphy et al., 1976).
Absorption of Iron in the Plant
The normal content of Fe in the tissue of vegetable crops is 50-300 mg/kg (ppm) in dry material (Zuang, 1982). Olsen et al. (1981) mentions that, in general, the content of iron required for a typical crop during the growing season is 5-10 kg/ha. The content of Fe+3 in many soils is much higher than this level although, as was mentioned, the problem with this ionic form is its low solubility (Chen y Barak, 1982, Olsen et al., 1981).
Plants have two different ways or strategies that they use to improve the availability of Fe+3 in aqueous soil solution:
Strategy I: The monocotyledons except grasses and the dicotylodans can lower the pH in their rhizosphere. Lowering the pH solubilizes Fe+3 and promotes it’s reduction to Fe+2. The solubiliezed Fe+3 is reduced to Fe+2 before it crosses the cellular membrane by the action of reducing proteins associated with the cellular membranes.
Strategy II: Grasses excrete phytosiderophores, non-protein amino acids that solubilize Fe+3 ions and form a Fe-phytosiderophore complex. The release of phytosiderophores is positively correlated with genotypic differences in resistance to iron chlorosis. Phytosiderophores also transport other cations such as Zn, Mn and Cu.
Fe Deficiency in Plants
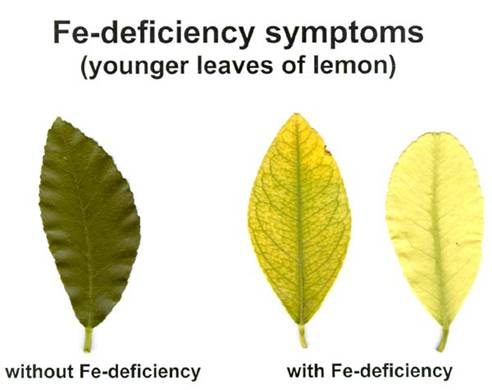
Fe deficiency symptoms:

New leaves growing point of plant become yellow to white or chlorotic without necrosis.

Youngest leaves become pale yellow. Brown areas develop around the main veins. Youngest leaves may become bleached almost white.
Symptoms start on younger leaves, the intercostal areas become chlorotic yellow, the veins remaining green.
At severe deficiency, the leaves may become nearly white, and the veins become chlorotic, too.
Newly developed leaves remain small.